Sleep Network Dynamics Underlying Flexible Memory Consolidation and Learning
Project Leader: Carmen Varela
One of the features that distinguishes mammalian intelligence is the ability to meaningfully extract, and flexibly integrate information into models of the world stored in distributed cellular networks of the forebrain. Flexible representations can accommodate noise and transformations in sensory input, encode statistical priors of features and allow inference beyond experienced examples. Hippocampal and thalamocortical networks provide a model system to study the computations and cellular implementation behind flexible learning. Episodic memories are initially acquired by the hippocampus and over time relevant information is integrated into neocortical schemas allowing for generalization and abstraction (Marr, 1971; Kumaran et al. 2016). This process leads to the consolidation of memory and flexible learning, and occurs mainly during resting states and sleep, when neuronal activity is largely driven by internal dynamics. In particular, the oscillations that dominate non-REM sleep are thought to organize and coordinate activity across multiple brain areas in order to facilitate this learning process (Wilson et al., 2015; Penagos et al., 2017).
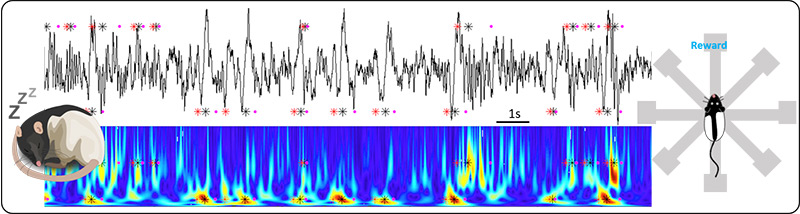
An oscillation that stands out because of its relevance to intelligence is the spindle oscillation. This 7-15Hz rhythm is driven by the thalamus, which then engages neocortical networks from primary sensory to higher order cortical regions, suggesting a fundamental function regardless of system modality. In humans, the number of spindles increases in the sleep following learning, and it is positively correlated with IQ scores as well as with performance improvements after sleep. Conversely, cognitive impairment is often associated with a decrease in spindles (Fogel & Smith, 2011; Wamsley et al., 2012; Lafortune et al., 2014).
Interestingly, we and others have observed that sharp-wave ripple oscillations (SWRs, 150-200Hz) in the CA1 region of the hippocampus are nested in individual spindle cycles during sleep. SWRs correspond to the reactivation of potential episodic memory traces. As the spindle oscillation progresses, SWRs occur nested in its cycles and this is thought to reinforce and help consolidate memories into neocortex; but how can cellular activity reinforce and transfer memories to neocortex while keeping them flexible to promote generalization? We will investigate this question by studying the correlation of thalamocortical cells with non-REM sleep oscillations in neocortex and hippocampus.
This project complements and extends ongoing work that investigates intelligent computations, such as those underlying interactions between different learning systems or sensory interpretation with tolerance to input transformations.